Physical chemical changes and properties color by number – Delve into the captivating world of physical and chemical changes, where the transformative power of color by number unveils the hidden secrets of matter. This exploration promises an illuminating journey, unravelling the fundamental concepts that govern the behaviour of substances around us.
As we embark on this adventure, we will witness how physical changes alter the form and appearance of substances without affecting their chemical composition. We will then delve into the realm of chemical changes, where substances undergo profound transformations, giving rise to entirely new compounds with distinct properties.
Physical Changes: Physical Chemical Changes And Properties Color By Number
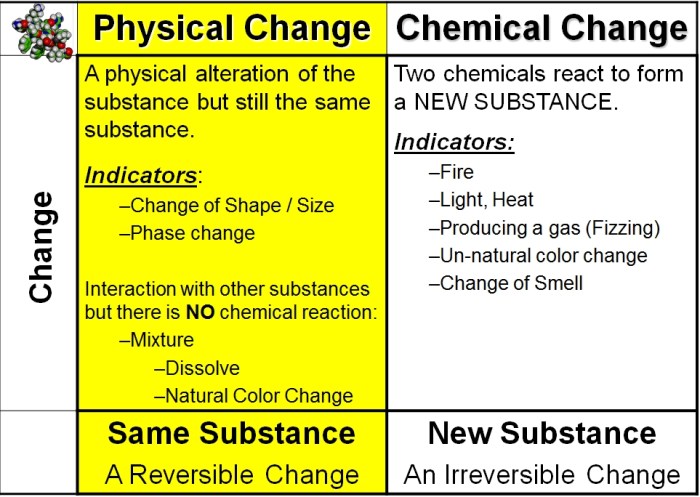
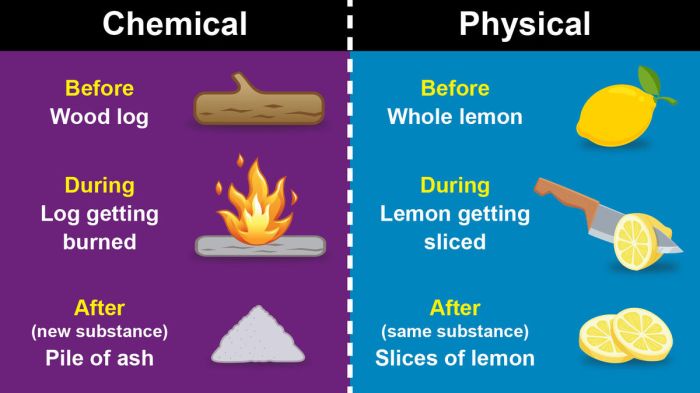
Physical changes are changes in the form or appearance of a substance without any change in its chemical composition. These changes are typically reversible, meaning that the substance can be returned to its original form.
Examples of physical changes include:
- Melting (solid to liquid)
- Freezing (liquid to solid)
- Boiling (liquid to gas)
- Condensation (gas to liquid)
- Sublimation (solid directly to gas)
- Deposition (gas directly to solid)
- Dissolving (solid or gas into a liquid)
- Crystallization (liquid to solid)
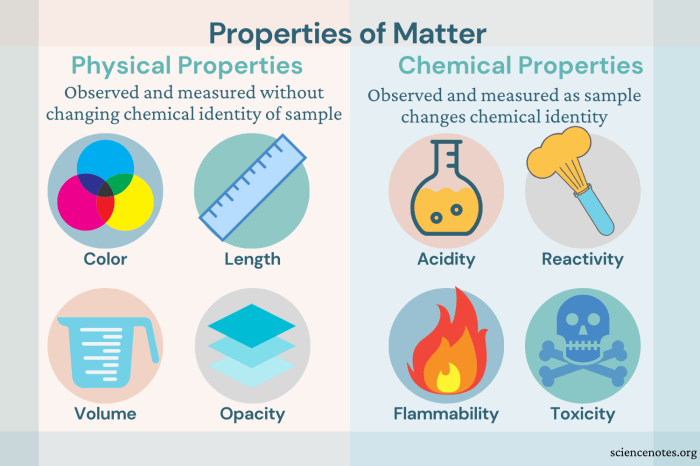
Physical changes do not affect the chemical composition of a substance, so the properties of the substance remain the same. For example, the melting point, boiling point, and density of a substance are all physical properties that remain the same regardless of whether the substance is in a solid, liquid, or gas state.
Chemical Changes
Chemical changes are changes in the chemical composition of a substance. These changes are typically irreversible, meaning that the substance cannot be returned to its original form.
Examples of chemical changes include:
- Burning (combustion)
- Rusting (oxidation)
- Digestion
- Photosynthesis
- Fermentation
- Cooking
- Electrolysis
- Nuclear reactions
Chemical changes affect the chemical composition of a substance, so the properties of the substance can change. For example, when iron rusts, it changes from a solid to a flaky powder and its strength and durability decrease.
Color by Number
Color by number is a technique used to create images by filling in numbered areas with the corresponding colors. This technique is often used to teach children about numbers and colors, but it can also be used to teach about physical and chemical changes.
For example, a color by number activity could be used to illustrate the process of melting and freezing. The activity could show a picture of a snowman in a solid state, and then the numbers could be used to fill in the areas of the snowman that are melting.
This would help children to understand how the snowman changes from a solid to a liquid state.
Applications
The concepts of physical and chemical changes and color by number can be applied in a variety of real-life situations. For example, the concept of physical changes is used in the food industry to preserve food. By freezing or canning food, the food can be prevented from spoiling.
The concept of chemical changes is used in the medical field to develop new drugs and treatments. By understanding how chemical changes occur, scientists can develop new ways to treat diseases.
Color by number can be used in a variety of educational settings. It can be used to teach children about numbers and colors, but it can also be used to teach about more complex concepts such as physical and chemical changes.
Additional Resources
- Khan Academy: Types of Chemical Reactions
- Encyclopædia Britannica: Physical Change
- Education.com: Color by Number Activities
Frequently Asked Questions
What is the key difference between physical and chemical changes?
Physical changes involve alterations in the physical form or appearance of a substance, while chemical changes result in the formation of new substances with different chemical compositions.
How can color by number be used to illustrate physical and chemical changes?
Color by number activities can visually represent the changes in substance properties, such as color, texture, and solubility, during both physical and chemical transformations.
What are some examples of real-life applications of physical and chemical changes?
Physical changes are utilized in processes like melting, freezing, and sublimation, while chemical changes underpin reactions like combustion, digestion, and photosynthesis.