The percent composition of a hydrate lab answer key unlocks the secrets of chemical composition, revealing the intricate relationship between water and ionic compounds. This guide delves into the intricacies of the lab, providing a comprehensive overview of the materials, procedures, and calculations involved in determining the exact composition of hydrated compounds.
Through a series of meticulously designed experiments, students embark on a journey of discovery, uncovering the fundamental principles of stoichiometry and the nature of chemical bonding. With each step, the answer key serves as an invaluable resource, guiding them towards a deeper understanding of the fascinating world of chemistry.
Percent Composition of a Hydrate Lab
The percent composition of a hydrate lab is a fundamental experiment in chemistry that demonstrates the concept of hydration and the determination of the empirical formula of a hydrate. It involves the quantitative analysis of a hydrate, a compound that contains water molecules bound to a salt in a specific ratio.
Lab Overview, Percent composition of a hydrate lab answer key
The purpose of this lab is to determine the percent composition of a hydrate by measuring the mass of water lost upon heating and the mass of the anhydrous salt remaining. The steps involved in conducting the lab include:
- Measuring the mass of a crucible and lid.
- Adding a weighed sample of the hydrate to the crucible.
- Heating the crucible and hydrate using a Bunsen burner until all the water has been driven off.
- Cooling the crucible and anhydrous salt and measuring the mass.
- Calculating the mass of water lost and the percent composition of the hydrate.
Materials and Equipment
The following materials and equipment are required for the lab:
- Hydrate sample
- Crucible and lid
- Bunsen burner
- Wire gauze
- Tongs
- Analytical balance
- Desiccator
Frequently Asked Questions: Percent Composition Of A Hydrate Lab Answer Key
What is the purpose of a percent composition of a hydrate lab?
The purpose of a percent composition of a hydrate lab is to determine the exact amount of water present in a hydrated compound.
What materials are required for a percent composition of a hydrate lab?
The materials required for a percent composition of a hydrate lab include a hydrate sample, a crucible, a balance, a Bunsen burner, and a desiccator.
What is the procedure for a percent composition of a hydrate lab?
The procedure for a percent composition of a hydrate lab involves weighing the hydrate sample, heating it to remove the water, and then weighing the anhydrous compound.
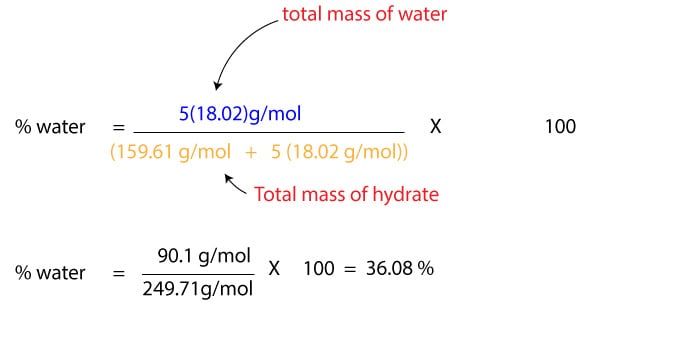
How do you calculate the percent composition of a hydrate?
The percent composition of a hydrate is calculated by dividing the mass of water lost by the mass of the original hydrate sample and multiplying by 100.


